Huntington’s Disease (HD) primarily affects a region deep inside the brain called the striatum. However, parts of the cortex are also known to be affected. The cortex is the outside layer of the brain with a walnut shape. This area has been less well studied, but we do know that it contributes to some of the variation in symptoms between people. For more information, check out this study (https://www.hdyo.co.nz/research/2024/7/21/dysfunction-in-the-middle-cingulate-cortex-is-associated-with-the-mood-symptoms-of-hd).
Along with increasing interest in the role of the cortex in HD, there has been a lot of interest in the role of brain support cells. Although it is primarily neurons (the brain cells that send electrical signals) that die in HD, there is growing evidence that some of the support cells might be dysfunctional, and that this dysfunction might contribute to the disease.
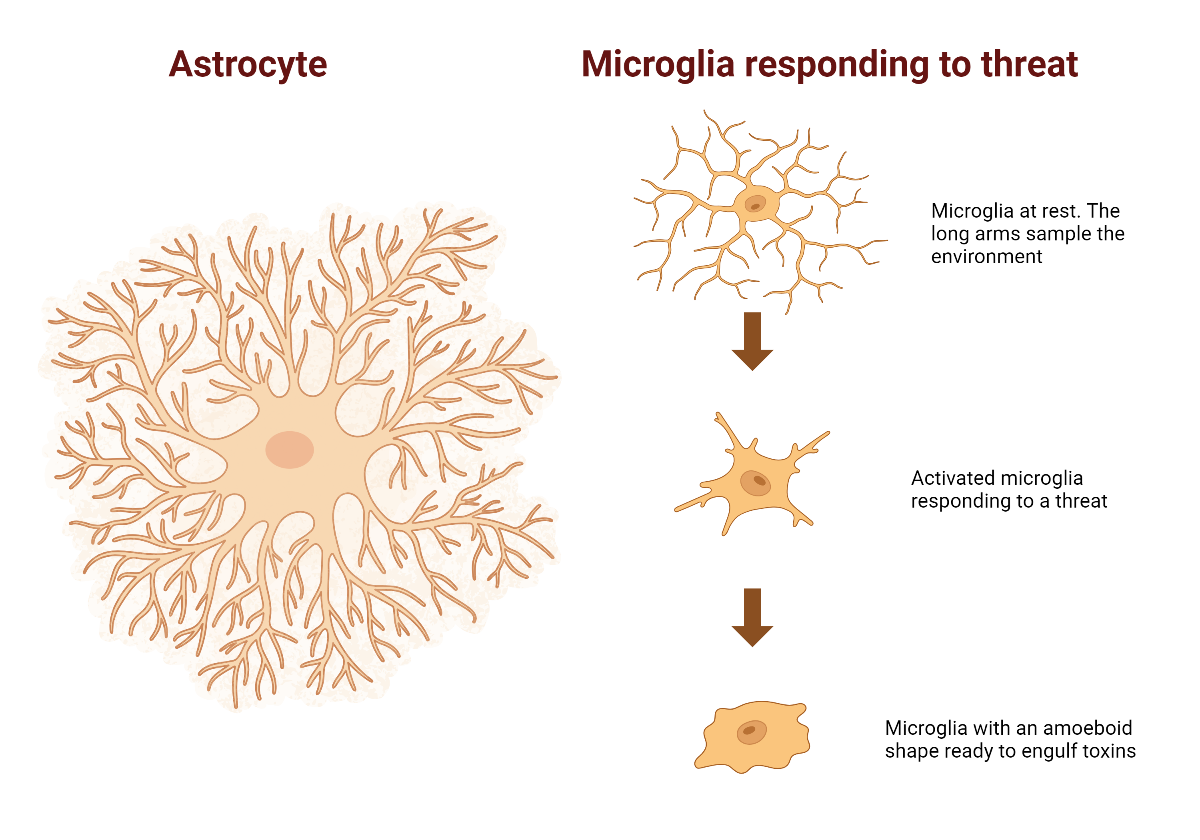
The most common support cells in the brain are called astrocytes, because they have a lot of fine branches and look a bit like a star. These cells have a lot of roles, from providing a scaffold for neurons to mopping up toxins. They also maintain a chemical balance in the liquid around neurons to make sure they can function optimally.
Microglia are the brains main immune cells. The cells are tiny and can move around the brain to the site of injury and infection. In a normal state they send out fine branches to sample the environment around them. When they detect a problem, they change shape so that they can respond.
Image created using BioRender.com
Both astrocytes and microglia contribute to the brain’s inflammation response. While inflammation is helpful for healing injuries, inflammation can cause problems if it goes on for too long. It has been hypothesised that excessive inflammation might be causing or contributing to the death of neurons in HD. Studies investigating this hypothesis had already been done at the Centre for Brain Research in the University of Auckland. Most had identified some issues with both astrocytes and microglia in different parts of the HD brain.
To extend this research, Adelie Tan, a scientist at the university, conducted a study on a part of the cortex called the Middle Temporal Gyrus (MTG). The MTG is located on the side of the brain above the ear. It is known to be only mildly affected in HD. This is helpful for research, because it means that most of the neurons are still alive when the patient dies of HD. This makes it easier to study the process that led to neuron death. By the time a region of the brain has mostly died, the situation often looks like mess, with many different changes. Untangling what came first can be very difficult.
To study the MTG in HD, Adelie and her team used samples from the brains of 29 people who had died of HD and 35 who had died without HD. These brains had been donated for research and are part of the Neurological Foundation Human Brain Bank.
This study used a method for screening large numbers of brains at the same time, called a Tissue Micro Arrays (TMA). The method involves drilling a tiny 0.2mm core out of each brain and placing them all in the same block of wax. Thin slices are then taken through the block so that there is a sample of each brain in every slice. Each slice is placed on a glass microscope slide. Different stains are then added so scientists can see different cells and cellular components.
Example of a Tissue Micro Array block. Each brown spot is a piece of brain tissue that has been drilled out of a human brain and placed inside the wax block. Slices are then taken from the top of the block, which allows samples from many human brains to be studied at the same time.
The TMA block used in this study had already been created and used in a previous study. That study looked at the neurons in the MTG of those with HD. This study aimed to extend our knowledge by looking at how the support cells in the MTG were functioning.
First, the team looked at astrocytes. Astrocytes are known to have specific differences in the HD brain which indicate that they are not doing their job properly.
The astrocytes in the MTC of the HD brains were found to have more of a scaffold protein known as GFAP. This protein helps to give astrocytes their shape. Excessive amounts are seen when the astrocytes band together and wall off a damaged area of the brain, essentially creating a scar. The amount of GFAP wasn’t associated with the severity of the disease, but it was associated with the amount of Huntingtin protein deposits present. Deposits of Huntingtin protein are the characteristic symptom of Huntington’s Disease.
The other markers of astrocyte function were all normal. This means that it is probably not astrocyte dysfunction that is causing the increase in GFAP in this part of the brain but may instead be the astrocytes responding to Huntingtin deposits. Previous studies have shown that Huntingtin protein ends up inside astrocytes, so this may be a way of cleaning it up. There was also no evidence that the astrocytes were replicating, which means that it was the existing astrocytes that were producing more GFAP.
Second, the team looked at microglia. Because different types of microglia do different jobs, several different stains were used to capture different types.
In the HD brains, the microglia were larger on average. Microglia were also present in different forms in the HD brain, particularly in shapes that respond to a perceived threat. A subset of HD brains also showed a second change. In these brains the microglia appeared to be replicating and increasing in number. This subset of HD brains belonged to people who had the longest (worst) forms of the Huntingtin gene and who died the youngest.
It is unclear what these microglial differences in the HD brain mean. However, proving that there are changes is the first step in finding out. Therapies that lowered levels of microglia in HD have had mixed results. It is possible that different groups of microglia affect the brain differently, so studies like this are an important first step in figuring out how to target the right ones.
In summary, this study provides an important contribution to our knowledge about how HD affects different parts of the brain. It is the first study to use a TMA from the cortex to look at astrocyte and microglia function in HD. Because this study focusses on a part of the brain only mildly affected by HD, it may help shed light on the mechanisms that become dysfunctional earliest in the disease. Each new piece of knowledge brings us closer to understanding how HD happens. In the process, it also brings us closer to curing the disease.